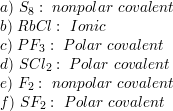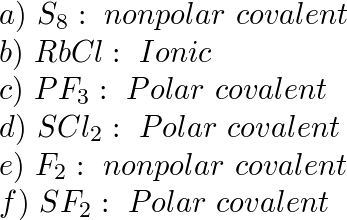Figure S8. Water molecules surrounding the nonpolar lutein isoprenoid... | Download Scientific Diagram

Facile Access to Polar-Functionalized Ultrahigh Molecular Weight Polyethylene at Ambient Conditions | CCS Chem
Developing new antiferroelectric and ferroelectric oxides and chalcogenides within the A2BX3 family | SpringerLink

Fig. S8.1: χ (2) eff across the Li K-edge comparison between systems... | Download Scientific Diagram

Polymerization-Induced Self-Assembly (PISA) Generated Cholesterol-Based Block Copolymer Nano-Objects in a Nonpolar Solvent: Combined Experimental and Simulation Study | Macromolecules

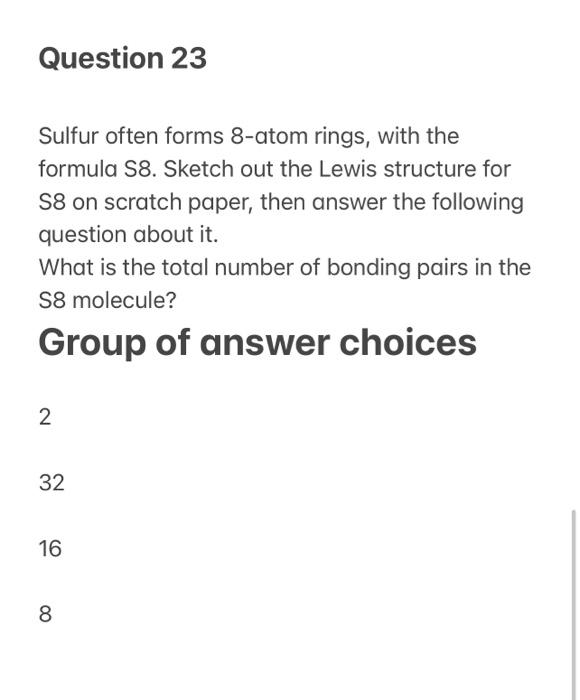
Measurement and Correlation of the Solubilities of Sulfur S8 in 10 Solvents | Journal of Chemical & Engineering Data
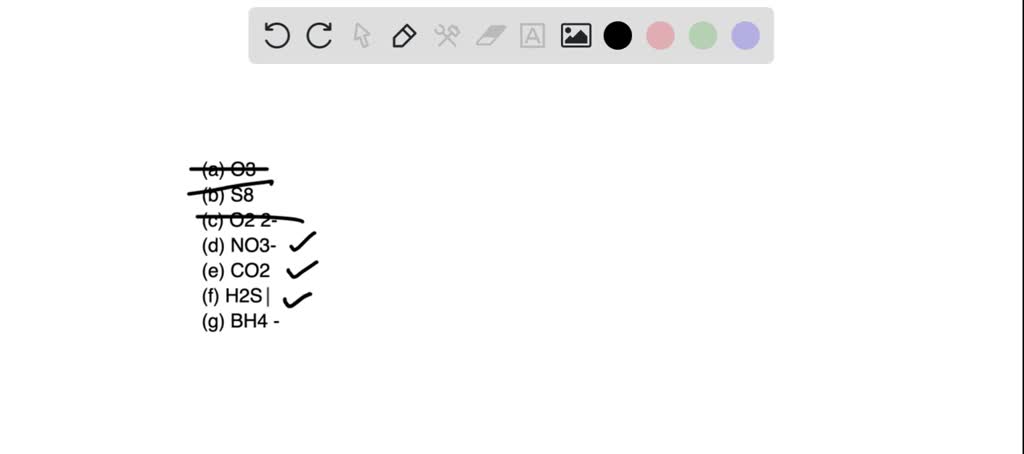
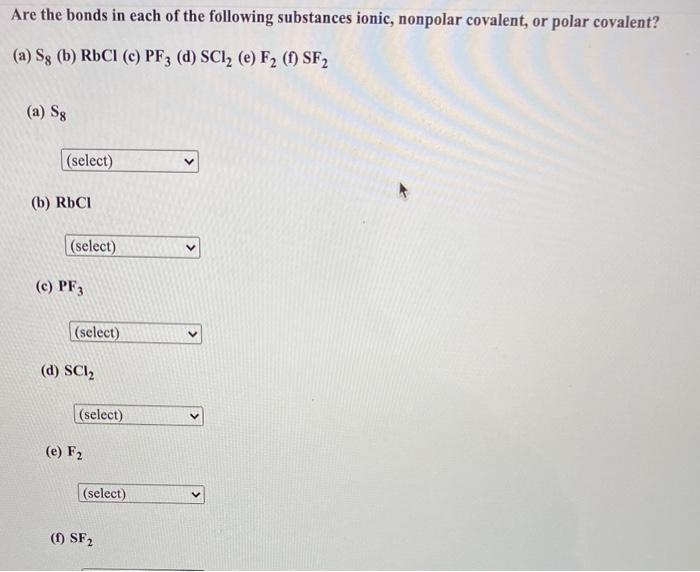
SOLVED: 22. Which of the following molecules or ions contain polar bonds? (a) O3 (b) S8 (c) O2 2− (d) NO3 − (e) CO2 (f) H2S (g) BH4 − My answers are:

Mechanism and energetics of O and O2 adsorption on polar and non-polar ZnO surfaces: The Journal of Chemical Physics: Vol 144, No 18

Amino Acid Specific Nonenzymatic Montmorillonite‐Promoted RNA Polymerization - Namani - 2021 - ChemSystemsChem - Wiley Online Library
Why does sulfur form S8 and not S6? Isn't the six-membered ring supposed to be more stable than the eight-membered ring. - Quora

Adsorption of neutral organic compounds on polar and nonpolar microplastics: Prediction and insight into mechanisms based on pp-LFERs - ScienceDirect

Polar–Nonpolar Transition-Type Negative Thermal Expansion with 11.1% Volume Shrinkage by Design | Chemistry of Materials

Cooperative evolution of polar distortion and nonpolar rotation of oxygen octahedra in oxide heterostructures | Science Advances
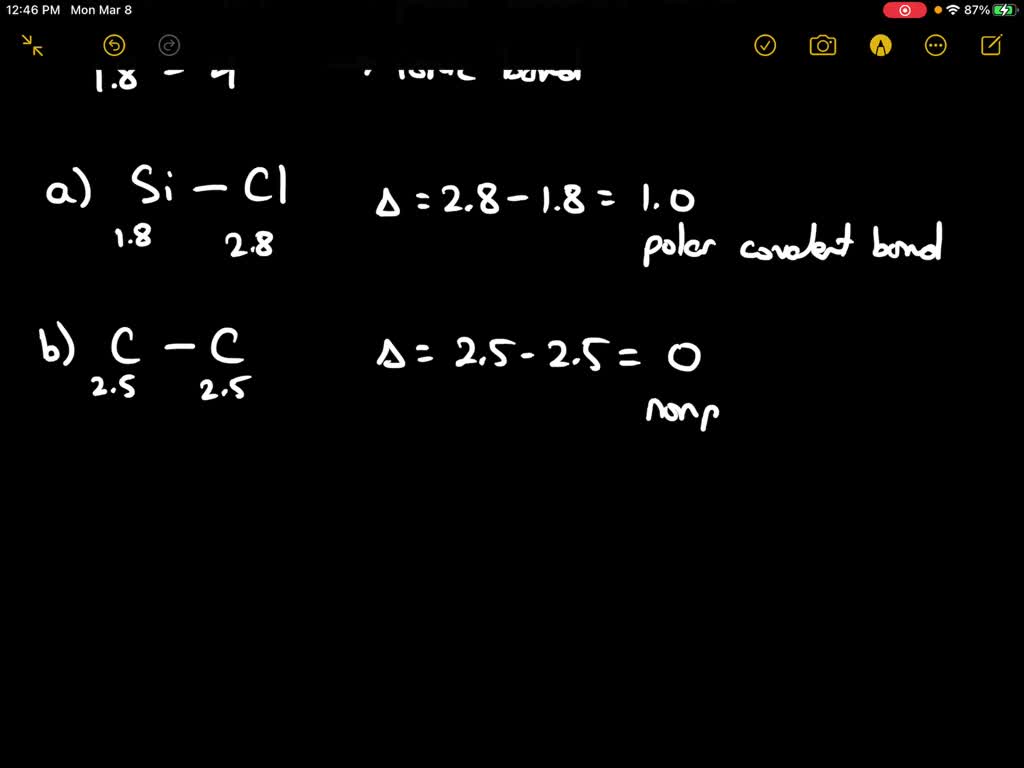
SOLVED: Calculate the electronegativity differences of the following and determine if they are largely ionic, non-polar covalent, or polar covalent? S8, CaCl2, SOCl2, NaF, CBr4, BrF, LiF and AsH3

22 | Which of the following molecules or ions contain polar bonds? O3, S8, O2 2-, NO3-, CO2, H2S - YouTube